Relating material properties to manufacturing performance and product quality

Supplies more than 70 global markets
GSK’s Dungarvan manufacturing site started operations in 1981 and is now one of the largest employers in the south east of Ireland. The site supplies more than 70 global markets with pain relief and denture care products. A centre of packaging excellence, the site boasts a committed workforce with technical, engineering, quality and operational expertise.
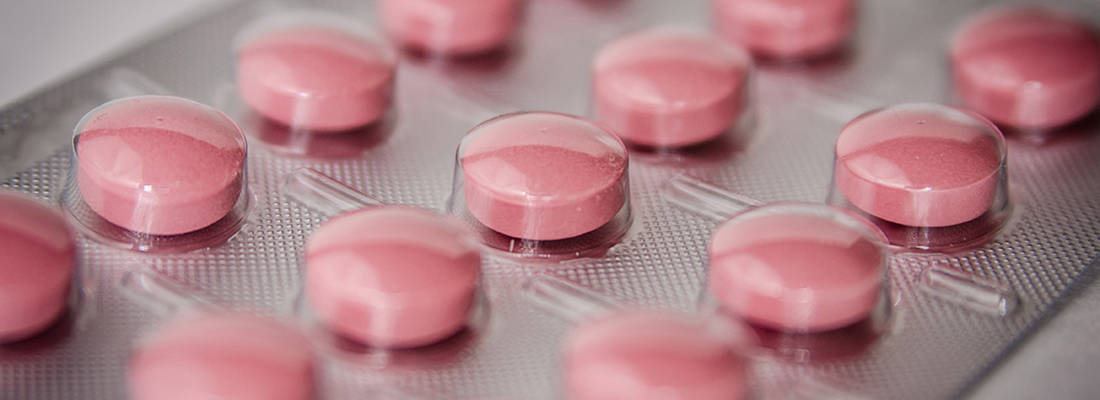
GSK Dungarvan manufactures high-volume products such as Panadol (6.5 billion tablets a year) for international export. The high-volume nature of the operations requires a consistent and reliable supply chain of active ingredients and excipients. While there are many potential suppliers for these ingredients, not every source is suitable for GSK’s products and manufacturing processes. Certain physico-chemical properties such as particle size, powder flow, porosity, crystal polymorphs, water sorption and dissolution are required for high throughput manufacturing and to ensure the quality of the finished product.
Suite of advanced characterisation techniques
The PMBRC Technology Gateway has a highly experienced team of solid-state pharmaceutical scientists backed up by academic staff that are actively involved in drug product formulation and materials characterisation research. Using a suite of advanced characterisation techniques, the Technology Gateway works with the GSK team to investigate the material properties and determine the ingredient sources most suitable for their products and manufacturing processes.
For example, the recent acquisition of an X-ray diffractometer with an environmental chamber (funded by the Enterprise Ireland Capital Infrastructure programme) allows the study of ingredient polymorphs (crystal forms) as a function of temperature and relative humidity. This in turn allows GSK to understand, optimise and trial materials most likely to best perform during manufacture and yield a good shelf life on storage.

1981
date operations founded
>70
global markets
1
direct-funded project
“The PMBRC offers GSK Dungarvan a local, highly professional and agile materials characterisation support, with timely data generation, thus helping GSK maintain and meet our business expectations. The PMBRC has allowed GSK conduct critical materials characterisation, supporting supplier introductions, along with root cause analyses investigations, ensuring ongoing supply of GSK Consumer Healthcare Products.”
Dr. Patrick Mooney
Raw Materials Technical Change Specialist, GSK (Dungarvan, Co. Waterford)
The GSK & PMBRC Technology Gateway partnership
Collaboration with the PMBRC Technology Gateway has allowed GSK Dungarvan to identify the best raw material sources for key products in their portfolio and has supported maintenance of the highest quality standards.